《结构化学》补充习题(化学专业)
第四章 分子对称性与群论初步
1. 填空题
(1) 四氢呋喃(C4H8O) 分子属于________点群。
(2) 乙烯分子属________点群。
(3) 分子中既不存在Cn轴,也不存在σ,则________(填可能或不可能)存在Sn。
(4) 对称元素C2与σh组合得到_________;Cn轴与垂直它的C2'组合得到________。
(5) 有一AB3分子,实验测得偶极距为零,且有一个三重轴,则此分子所属点群是________。
(6) 有两个分子,N3B3H6和C4H4F2,已知分子都是非极性分子的,且为反磁性的,N3B3H6几何构型________,点群________; C4H4F2几何构型________,点群________。
(7) CH2=C=O 分子属于___________点群;CH2=C=CH2分子属___________点群;CH2=C=C=CH2分子属___________点群。
(8) 既有偶极距又有旋光性的分子必属于________点群。
(9) NF3分子属于________点群,该分子是极性分子,其偶极距向量位于_________上。
(10)椅式环己烷(C6H12)分子属于____________点群, SF6分子属于____________点群。
(11)某分子具有一个二重轴、一个对称面和一个对称中心,该分子属于______点群。
(12) 两个C2轴相交,夹角为2p/2n,通过交点必有一个_______次轴,该轴与两个C2轴_________。
(13) 交角为45°的相邻两镜面的交线是_______轴。
(14)在D5点群中,两个二重轴之间最小的夹角是________________________。
(15) 两个对称面相交,夹角为2p/2n,则交线必为一个_______次轴。
(16) 在C2v点群中,两个对称面之间的夹角是_____________________。
(17)在下列空格中写上“有”或“无”。
分子所属点群 | Ci | Cnv | Dn | Td | Dnd |
偶极距 旋光性 |
|
|
|
|
|
2. 选择题
(1) 下列说法正确的是: ················································································· ( )
A、凡是八面体配合物一定是属于Oh点群;
B、凡是四面体构型的分子一定属于Td点群;
C、异核双原子分子一定没有对称中心;
D、在分子点群中对称性最低的是C1点群,对称性最高的是Oh点群。
(2) 下列分子中偶极距不为零的分子是:···························································· ( )
A、BeCl2 B、BF3 C、NF3 D、CH3+
(3) 下列分子中偶极距为零的是:····································································· ( )
A、NF3 B、NO2 C、PCl5 D、BCl3
(4) 下列分子中偶极距不为零的是:··································································· ( )
A、SO3 B、SF6 C、H2O2 D、CH2Cl2 E、SO42-
(5) 下列分子具有偶极距,而不属于Cnv点群的是:············································ ( )
A、H2O2 B、NH3 C、CH2Cl2 D、CH2=CH2
(6) 环形S8分子属于D4d点群,分子中包含轴次最高的旋转轴是:···························· ( )
A、C4 B、S4 C、S8 D、C8
(7) 下列说法正确的是: ·················································································· ( )
A、分子中各类对称元素的完全集合构成分子的对称群;
B、同一种分子必然属于一个点群,不同种分子必然属于不同的点群;
C、分子中有Sn轴,则此分子必然同时存在Cn轴和σh;
D、镜面σd一定也是σv。
(8) 如果某分子有S6,那么也必然包括: ······························································( )
A、C6, σh B、C3, σh C、C3, i D、C6, i
(9) B2H6所属点群是: ··················································································( )
A、C2v B、D2h C、C3v D、D3h
(10)与NH3分子属于不同点群的分子是: ························································( )
A、BF3 B、CH3Cl C、CHCl3 D、PCl3
(11)与H2O分子不同点群的分子是: ·····························································( )
A、吡咯 B、吡啶 C、甲醛 D、CO2
(12)下列分子属于Td点群的是: ·····································································( )
A、SO42- B、PO43- C、ClO4‑ D、SiH4
(13)下列分子中属于 D3群的是: ········································································( )
A、BF3 B、NH3 C、部分交错式乙烷 D、交错式乙烷
(14)下列命题中正确者为: ················································································( )
A、含不对称 C 原子的分子具有旋光性
B、无不对称 C 原子的分子无旋光性
C、不具有反轴对称性的分子在理论上有旋光性
D、凡是有镜面的分子都不具有偶极矩和旋光性
(15)下列分子中既具有偶极矩又具有旋光性的是:···················································( )
A、H2O B、CO2 C、CH4 D、氟氯代甲醇
(16) 属于那一点群的分子可能有旋光性?····························································( )
A、Cs B、D¥������h C、 Oh D、Dn
(17) 下列配合物中,具有旋光异构体的是····························································( )
A、[Pt(NH3)2Cl2] B、[Co(NH3)4Cl2]Cl C、[Co(en)3]Cl3 D、[Pt(NH3)ClBrPy]
3. 问答题
(1) 指出下列分子的点群、旋光性和偶极矩。
①CH2=C=CH2; ② 交叉式二茂铁; ③重叠二茂铁;④反式-1,3二氯环丁烷; ⑤间二氯苯;
⑥CH2=CHCl; ⑦CH2Cl2; ⑧溴代吡啶 ⑨环戊二烯负离子 ⑩ 1,3,5 -三氯苯
(2) 写出下列分子所属点群的记号及有无偶极矩(北京大学,1994年研究生试题):
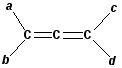 ① a=b=c=d ② a=b≠c=d
① a=b=c=d ② a=b≠c=d
③ a=d≠b=c ④ a≠b≠c≠d
(3) 指出下列分子所属点群,并判断旋光性和偶极矩。
H2O; NH3; SO3; HF; H2O2; C60;SO2; N2O; XeF4; IF5;B(OH)3;O3;CCl4; I3-
1